| Definition | Chikungunya virus infection | ||
|---|---|---|---|
| Disease classification | Class 3 infectious disease (disease code: A92.0) | ||
| Pathogens |
Togaviridae Alphavirus Chikungunya virus (Positive single-stranded RNA virus) |
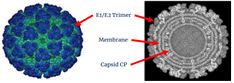
|
|
| Major vaccine antigens | Chikungunya structural polyprotein (C, E3, E2, 6k, E1) | ||
| Reservoir | Aedes ( Aedes albopictus or Aedes aegypti ) | ||
| Route of infection | Aedes -> People | Infection is spread through the bite of an vector mosquito infected with the Chikungunya virus (human-mosquito-human transmission).It is presumed that there is a possibility of transmission through blood in the case of blood transfusions, organ transplants, and syringe cuts. Cases of vertical infection have been reported | |
| Domestic occurrence | Over the past five years, there are 3 cases in 2018, 16 cases in 2019, 1 case in 2020, and 6 cases in 2022 (tentative). | ||
| Overseas occurrence | First report | The first outbreak occurred in 1952 in the Makonde Plateau of Tanzinia, Africa. At that time, Chikungunya virus was isolated for the first time from the patient's serum, and it later became prevalent in sub-Saharan Africa. | |
| Occurrence trend |
(1963-2005) More than 140,000 cases occurred in India (2006~2007) Cases occurred in Africa, Asia, and Italy (2009) Virus outbreaks reported in Indonesia, Thailand, and Malaysia (2013~) Spread to the Caribbean, North America, and South America (2020) Multiple reports from Pakistan, India, Brazil, and the Caribbean |
||
| Risk areas | t occurs mainly endemic in Africa and Asia. In particular, countries around the Indian Ocean and Southeast Asia are considered risk areas. | ||
| Overseas inflow | There have been many cases of infection from overseas. In particular, most cases of infection occurred after visiting Southeast Asia. | ||
| Incubation period | 1-12 days (Average: 3-7 days) | ||
| Clinical symptoms |
|
||
| Fatality rate | The mortality rate is extremely low, and death cases are mainly observed in elderly people. | ||
| Diagnosis | Detection of specific genes in samples (blood, body fluids, etc.) (Real-time RT-PCR) | ||
| Treatment | There is no specific treatment commercially available worldwide. Symptom-based symptomatic treatment is best | ||
| Prevention |
|
||
| Definition | Acute, febrile and viral zoonotic infectious diseases caused by Nipah virus infection2)2) | ||
|---|---|---|---|
| WHO Disease classification | ICD-10 B33.8 (other specified viral diseases) | ||
| Pathogens |
(Paramyxoviridae) (Henipavirus) (NIpah virus) (Negative single-stranded RNA virus) |
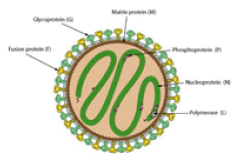
|
|
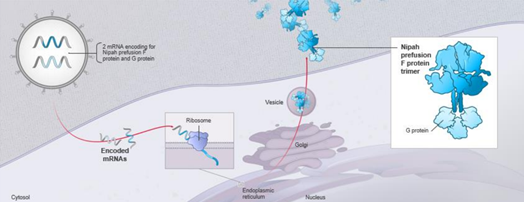
| Major vaccine antigens | Nipah virus glycoprotein (G, F) | ||
| Reservoir | Infected animals (fruit bats of the Pteropodidae and Pteropus genera, pigs, etc.), food contaminated with bodily fluids of infected animals, and patients in direct contact | ||
| Route of infection | Direct contact with virus-infected natural hospitals (e.g., fruit bats, pigs, etc.), direct contact with patients | ||
| Consuming food (e.g., palm sap or fruit) contaminated with bodily fluids from virus-infected animals | |||
| Domestic occurrence | None | ||
| Overseas occurrence | First report | The first case identified in Malaysia in 1998 | |
| Occurrence trend | Since it first case occurred in Sungai Nipa village, Malaysia in 1998-1999, its sporadic outbreaks have occurred in the Nipa belt region, including Bangladesh, India, the Philippines, and Singapore3)3) | ||
| Risk areas | Bangladesh, India, Malaysia, Philippines, Singapore, etc. | ||
| Overseas inflow | None | ||
| Incubation period | About 4 -14 days | ||
| Clinical symptoms |
|
||
| Fatality rate | 40-70% (depending on surveillance capacity in epidemic areas) | ||
| Diagnosis | Specific gene detection (real-time RT-PCR) and viral antigen-antibody reaction test in specimens (blood, body fluids, etc.) | ||
| Treatment | There are no specific treatments commercially available worldwide. Conservative treatment should be performed, and the antiviral drug ribavirin should be used when this virus is prevalent. | ||
| Prevention |
|
||

| Definition | Acute febrile and hemorrhagic disease caused by Lassa virus infection1)1) | ||
|---|---|---|---|
| Disease classification | Class 1 infectious disease (disease code: A96.2) | ||
| Pathogens |
(Arenaviridae) 라싸 (Lassa virus) (Single-stranded RNA virus divided into two segments) |
|
|
| Major vaccine antigens | Envelope GPC(GP1, GP2) glycoprotein | ||
| Reservoir | Mastomys natalensis among rodents | ||
| Route of infection | Animal → People |
Direct or indirect contact with infected rodents (rats)/inhalation of rodent droppings (urine, feces) (Ingestion) Ingestion of food contaminated with rats or rat droppings (Contact) Exposure of broken skin or mucous membranes to rat feces absorbed into the soil (Inhalation) Inhalation of aerosols generated during cleaning of floors contaminated with rat droppings |
|
| People → People |
Contact with blood or body fluids of Lassa fever patients or deceased (Contact) Direct contact with the patient's blood or body fluids on the wounded skin or mucous membrane (Contact) Sexual contact with an infected patient (Contact/inhalation) Infection spread through exposure during medical treatment or procedures in a medical environment |
||
| Domestic occurrence | None | ||
| Overseas occurrence | First report | An outbreak was reported in Lassa area, Borno State, Nigeria in 1969. | |
| Occurrence trend | In West Africa, outbreaks occur during the dry season (November to May) and occur sporadically throughout the year. | ||
| Risk areas | Benin, Ghana, Guinea, Nigeria, Liberia, Mali, Sierra Leone, Burkina Faso, Côte d'Ivoire, Togo Central African Republic (others in West Africa) | ||
| Overseas inflow | 1969-2016, 33 cases in 9 countries (UK(13), US(8), Germany(5), Netherlands(2), Canada(1), Israel(1), Japan(1), Sweden(1), South Africa(1)) | ||
| Incubation period | About 2 -21 days | ||
| Clinical symptoms |
|
||
| Fatality rate | About 1-3% of infected people, 15-20% of hospitalized patients* Varies depending on the level of the health care system in each country (fatality rate during the 2015-2016 epidemic in Nigeria was 32.6%) | ||
| Diagnosis | Detection of specific genes in samples (blood, body fluids, etc.) (Real-time RT-PCR) | ||
| Treatment | There is no specific treatment commercially available worldwide (symptomatic treatment). However, antiviral drugs (ribavirin) are known to be effective when administered in the early stages of symptoms. | ||
| Prevention |
|
||
| Animal modesl for Lassa virus | Pros | Cons |
|---|---|---|
|
Murine Models Murine Models(Natalensis mastomys, IFNAR-/-, Chimeric IFNAR-/-B6, IFNαβ/γR-/-, STAT1-/-, CBA, HHD, etc) |
|
|
|
Guinea Pig Models (Strain 13-inbred, Hartley-outbred 등) |
|
|
|
Non-Human Primate Models (Cynomolgus macaques, Rhesus monkey, Marmoset, Squirrel monkeys 등) |
|
|
| Definition | Acute febrile illness caused by dengue virus infection11)2) | ||
|---|---|---|---|
| Disease classification | Class 3 infectious disease (disease codes: A90.0, A91.0) | ||
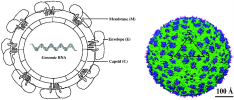
| Pathogens |
(Flaviviridae) (Flavivirus) (Dengue virus) (Benign single-stranded RNA virus) |
|
|
| Major vaccine antigens | structural proteins(Premembrane(prM), Envelope(E)) | ||
| Reservoir | Aedes mosquitoes (Aedes albopictus or Aedes aegypti), humans | ||
| Route of infection | Aedes mosquito ↔ People |
Infection is spread through the bite of a vector mosquito infected with the dengue virus. The virus can be transmitted to vectors (mosquitoes) by sucking blood from a person infected with the dengue virus and suffering from a persistent fever. |
|
| People → People | Blood transfusion, sexual contact, placenta, breast milk, etc. | ||
| Domestic occurrence | It was designated as a statutory infectious disease in 2000, and there have been no domestic cases of it. | ||
| Overseas occurrence | First report | AIt is difficult to know the exact time of the outbreak of the dengue virus, but it has existed for so long that there is a book published during the Jin Dynasty (265-420) in China. | |
| Occurrence trend | IIt occurs in more than 100 countries around the world, and the endemic area is very widespread, mainly in tropical and subtropical regions, up to 35° north and south latitude based on the equator. Because mosquitoes that spread dengue fever mainly breed in stagnant water, the number of patients increases rapidly during the rainy season. | ||
| Risk areas | |||
| Overseas inflow | Domestic reporting status due to overseas inflow: 227 people on average from 2013 to 2019, 43 people in 2020, 3 people in 2021, 104 people in 2022. | ||
| Incubation period | 5-7 days | ||
| Clinical symptoms |
|
||
| Fatality rate | 1% if treated early, about 20% if treated late | ||
| Diagnosis | Detection of specific genes in samples (blood, body fluids, etc.) (Real-time RT-PCR) | ||
| Treatment | There is no specialized treatment worldwide; thus, symptomatic treatment is best. | ||
| 예방 |
|
||
| Animal modesl for Degue virus | Pros | Cons |
|---|---|---|
|
Murine Models (C57BL/6 and BALB/c mice, AG129 mice, Humanized mice) |
|
|
| NHP (Rhesus macaques, Cynomolgus macaques, Marmoset, Bonnet macaque, Chimpanzee) Models |
|
|
|
Swine Models (Yucatan miniature pig) |
|
|
| Tree shrew Models |
|
|